Recent events have highlighted that infectious diseases still pose a significant threat to human health and the outlook for climate change is worrying for possible new risks. The research effort on infectious agents is underpinned by emerging pathogens, also by the threats of epidemics and pandemics responsible for high morbidity and mortality rates. Also, the possibility of using pathogenic microorganisms as agents of bio-terrorism strengthens the development of new effective therapeutic strategies. Finally, the importance of the microbiota opens up new perspectives for improving the fight against certain infections and in particular also for longevity.

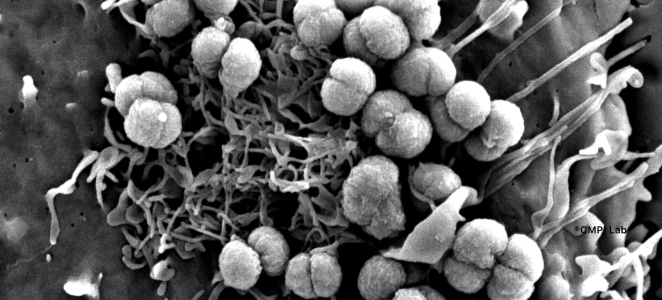

We are interested in the early stages of infection at the molecular and cellular levels. Our research focuses on the cellular mechanisms hijacked by pathogens to adhere to and invade host cells. The pathogens have developed numerous strategies to usurp the host’s molecular machinery in order to evade the immune response and to ensure effective infection. Autophagy is a ubiquitous process involved in many phenomena including immunity. Certain pathogens divert this mechanism to develop their replicative niche, to obtain a source of membranes and nutrients. We want to understand these mechanisms by studying pathogens such as norovirus or enteric and respiratory bacteria, even bacterial toxins, which develop different strategies targeting this cellular process.
The relationships between autophagy and probiotics and in particular lactic acid bacteria are still poorly understood and we are therefore exploring this new scientific field.
We are developing an interdisciplinary approach combining bacterial genetics, cell and molecular biology, biochemistry and biophysics to study how pathogens interact with the host’s cell surface, how the cell responds to this attack and how pathogens hijack this response. The goal is to identify new molecular targets to develop new therapeutic tools that will be used to inhibit infection.
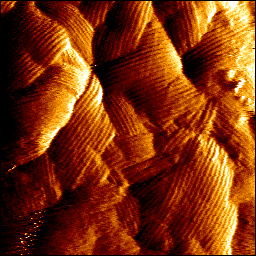



In particular, we are interested in the mechanical properties of membranes. How can they regulate the molecular interactions influencing the cellular response during an intercellular interaction between a microbe and a host cell? To do this, we have developed technological tools based on nanosciences and in particular near-field microscopy and super-resolution biophotonic microscopy, including in correlation. We use atomic force microcopy (AFM) in force spectroscopy studies to measure interactions between single molecules, for elasticity measurements of living cells or tissues. Fluorescence nanoscopy approaches can identify molecules in cells. We have thus established for the first time the triple correlation measurement between the microscopy modes, the Stiffness tomography to measure the elasticity of intracellular elements, and the automation of measurements in AFM in a robust way with correlation and elasticity measurements. Within European consortia, we are also developing standardization methods on cells and tissue.
The team welcomes biologists, doctors, pharmacists and physicists. Members are involved in teaching and scientific popularization. We enjoy hosting invited scientists for series of experiments or to collaborate for synergistic exchanges, so do not hesitate to contact us and come and discuss around dishes (including vegans) to discuss Science, biodiversity preservation or the latest sports results !!!




Cell Physics and surface signalling linked to host-pathogen interactions
- Biomechanics of Listeria monocytogenes–host cell interaction (with P Cossart Inst. Pasteur Paris)
- Biophysics of Trans-Endothelial Macroapertures induced by bacterial toxins (with E Lemichez Inst. Pasteur Paris)
- Neisseria interaction with plasma membrane: adhesion forces (With S Bourdoulous Inst Cochin)
- Signaling associated to the interaction of Yersinia to the cell surface
- Imaging at high speed membrane dynamics and protein/lipid interactions
- Biophysics of host-pathogen interaction at the nanometric scale
- Structure and dynamics of SNARE calcium-sensitive oligomers for synaptic vesicle transmission (with JE Rothmans Yale Univ)
- Nano-mechanics response measurements of cells and tissues in cancer (EU ITN consortium)
- Towards automation for nano-mechanics characterization of biological samples in diseases
Autophagy & Infection
- Autophagy hijacking during vacuolar infection phase: Yersinia
- Role of autophagy as a response to membrane damages: Shigella and Coxiella
- Role of autophagy in cellular responses to norovirus infection (G. Muharram)
- Characterization of autophagy regulation by Lactic Acid Bacteria both in vitro and in vivo (C. Daniel)
Technologies and Developments
- Atomic Force Microscopy and super-resolution fluorescence microscopy correlative method: Ciczora et al 2019 Biol Cell
- CLAFEM: Correlative Light Atomic Force Electronic Microscopy : Janel et al 2017 Methods in Cell Biology
- CLEM: Correlative Light Electron Microscopy on host-pathogen interaction Ligeon & al 2015 Methods
- Stiffness tomography of intracellular organelles : Roduit et al 2008,2009 Biophys J, Janel et al Nanoscale 2019
- AFM automation: Dujardin et al PLoS ONE 2019
- AFM Measurement standardization for cells and tissues: Schillers et al 2017 Sci Rep
Collaborations
- Cytoadhesion of Plasmodium falciparum-infected erythrocytes, ultrastructural characterization and elastic properties of knobs studied with AFM
- Ultrastructural analysis of osteoblasts mineralization
- Characterization of hydrophobins from Aspergillus fumigatus
- Adhesive properties of Staphylococcus aureus

